A test based on biomarkers aims to speed up diagnosis and enable prompt treatment.
A first-of-its-kind blood test that uses biomarkers to distinguish bipolar disorder from depression could slash the time it takes to get an accurate diagnosis from years to weeks, according to the company that developed the test — but some scientists have raised concerns about its validity.
The test uses biomarkers related to RNA editing to diagnose the condition and has been available in France since March and in Italy since October 2023, having been granted regulatory approval in both countries.
https://www.nature.com/articles/d41586-024-03616-7
However, some researchers are concerned about the small size of the trials the test is based on and the lack of independent verification of the studies. The test’s developer, the French start-up Alcediag in Montpellier, says that its trials are valid and reproducible.
The row highlights a wider discussion about the potential of biomarkers — biological characteristics that can indicate a particular medical state — to enable earlier diagnosis and more personalized treatments for psychiatric disorders.
“There is a role for investigating biomarkers,” says Suresh Sundram, a psychiatrist at Monash University in Melbourne, Australia. “But it is a very fraught area.”
Slow diagnosis
Bipolar disorder, a spectrum of conditions characterized by mood swings that alternate between mania and depression, is difficult to diagnose. About 40 million people globally live with the illness, and the diagnostic process, which often involves multiple sessions with a psychiatrist, takes an average of seven to ten years, during which time people are often misdiagnosed with conditions such as depression and given inappropriate or ineffective treatments.
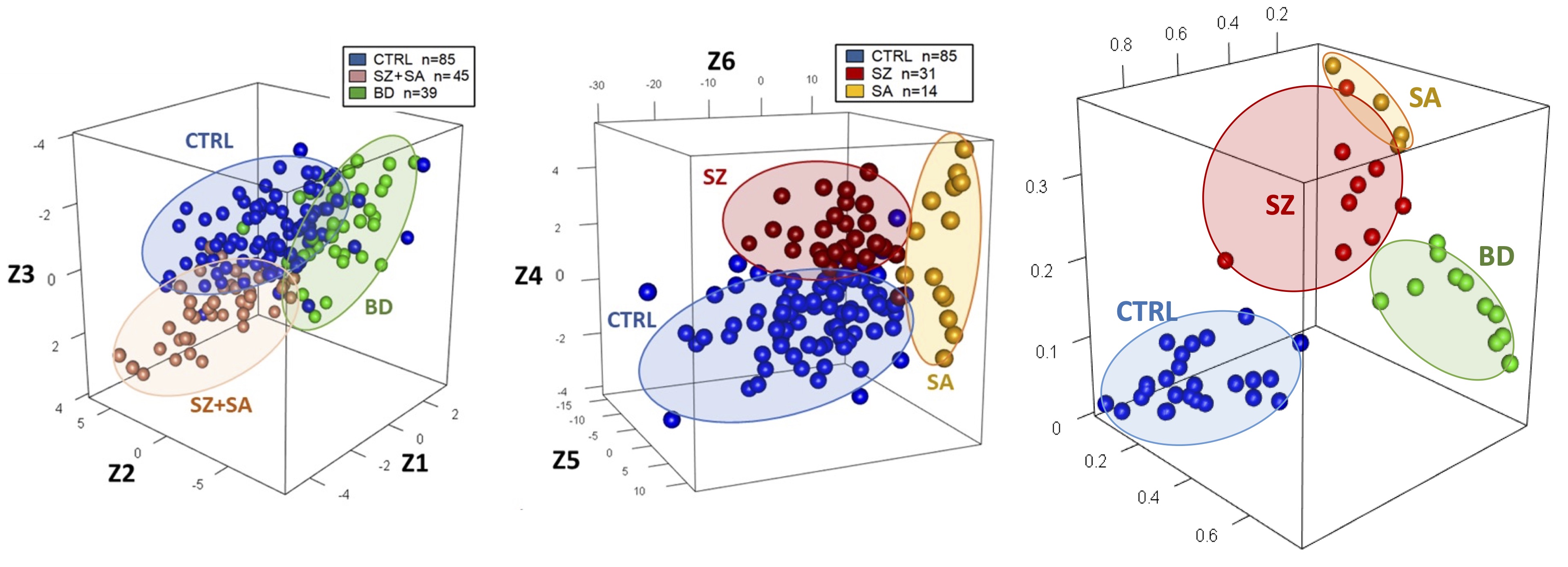
Alcediag hopes to alter this grim landscape. The company says its €900 (US$980) blood test, EDIT-B, can help to distinguish bipolar disorder from depression using biomarkers. EDIT-B differentiates between the two by measuring subtle differences in RNA editing — a regulatory process that alters various cellular mechanisms, including the expression of genes, which in turn affects neurological functioning.
Several studies have suggested that differences in RNA editing could play a part in autoimmune diseases and cancer, as well as in psychiatric conditions. In preliminary research, scientists at Alcediag identified distinct patterns of RNA editing affecting eight genes that seem to differ between healthy people and those experiencing depression. Among the depressed patients, six of these genes also show variations that distinguish people with depression from those with bipolar disorder. These differences produce a unique combination — or signature — of biomarkers that the company says it discovered using an artificial intelligence (AI) algorithm it developed.
“We have a signature for those with depression, a signature for the controls, and … one for bipolar,” says Dinah Weissmann, co-founder and chief scientific officer of Alcediag. In a 2022 study involving 410 participants, the algorithm distinguished between the 160 people with depression and the 95 individuals with bipolar disorder with high accuracy1.
About 80 people have used EDIT-B since its commercialization in France and Italy, says Weissmann, and feedback has been positive so far. She cites the anecdotal report of a person who, after receiving a positive test result, said they changed to more effective medication. “The patient wrote to their doctor: ‘It’s great, I’m back on my feet. I’m living normally again,’” says Weissmann.
Potential risks
For many people with bipolar disorder, a faster, more precise diagnosis would allow them to access “the right medication, at the right time”, says Marion Leboyer, a psychiatrist and executive director of the FondaMental Foundation, a research organisation based near Paris.
On the other hand, if a blood test gives an incorrect result, there is a risk that disorders could be misdiagnosed or overlooked, says Boris Chaumette, a psychiatrist at the French National Institute of Health and Medical Research in Paris.
There is no indication that an EDIT-B result has led to an incorrect diagnosis. But Chaumette and others are concerned about some of the methodology of studies used to demonstrate the EDIT-B test’s effectiveness. He points to “inherent limits” to the 2022 study with 410 participants. “You take a data set with many variables and not many patients, and you ask an algorithm to classify people. It will inevitably find things that classify them, it will inevitably identify commonalities,” he says. “In fact, what you observe could be the effect of treatments.”
He adds that, apart from the control group, every person involved in Alcediag’s studies was taking medication (as is often the case with psychiatric research). These drugs could affect the levels of some biomarkers, says Sundram, “so the algorithm could pick up the effect of the medicine”.
Weissmann says that the study participants with bipolar disorder and those with depression were taking a range of different medications, so if the algorithm was distinguishing people on the basis of their treatments, it would have classified them according to therapeutic classes. Stable patients — those who had a diagnosis but were not experiencing symptoms at the time of the study — also had a different biomarker signature from the control group, she adds, indicating that their medication suppressed symptoms without affecting markers of the underlying illness. She says that Alcediag’s studies have involved hundreds of participants, and that the company is conducting a new clinical trial with 436 patients; they expect to publish the results next year.
Replication questions
Certain aspects of Alcediag’s studies make it difficult for independent researchers to verify the work, says Chaumette. The algorithm and its underlying code haven’t been shared, and follow-up studies run after the initial 2022 trial have used slightly different versions of the test. In a study the firm published this year, for example, it had removed one biomarker from the initial combination and added three new ones2.
This issue led the French National Authority for Health (HAS), an independent body that assesses health products, to reject Alcediag’s application for people taking the test to be reimbursed by the French health authorities. “There were variations in performance among the three versions, and we lacked the rationale to explain why this version was chosen and not another,” says Cédric Carbonneil, who heads the HAS department responsible for assessing new medical devices and procedures. It is not unusual for companies at early stages of development to initially have their applications denied, and the HAS expects Alcediag to reapply, he adds. Alcediag says that it made changes to the biomarkers to improve the test’s performance, and that it hasn’t shared the algorithm for commercial reasons.
Chaumette says he would have liked to see larger studies backing up the test before it was rolled out to patients, and he hopes Alcediag will make its technology available to independent groups to allow them to replicate the findings. “If you commercialize it rapidly and patent everything without sharing anything, then it becomes opaque.”
doi: https://doi.org/10.1038/d41586-024-03616-7
References: Salvetat, N. et al. Transl. Psychiatry 12, 182 (2022).
Este estudo conduzido com brasileiros publicado na revista Psychiatry Research foi também divulgado https://www.sciencedirect.com/science/article/pii/S0165178123003724?via%3Dihub representa um avanço nesse sentido. Assinado por pesquisadores da Universidade Federal de São Paulo (UNIFESP), o trabalho descreve um painel de biomarcadores sanguíneos – relacionados com o mecanismo de edição de moléculas de RNA – que possibilitou discriminar com precisão pacientes com o transtorno bipolar ou com depressão. O resultado, segundo os autores, abre caminho para a criação de um exame de sangue que poderia dar suporte ao diagnóstico clínico.
A pesquisa é fruto de uma parceria com cientistas franceses ligados ao Centre National de la Recherche Scientifique (CNRS) e com a empresa de medicina personalizada ALCEDIAG. O grupo da UNIFESP é apoiada pela FAPESP por meio de seis projetos [22/00527-8; 20/01107-7; 19/13112-8; 19/08287-3; 17/02413-1; 14/50891-1].
Edição de RNA
Embora exista a tendência de elevar o DNA como protagonista quando se tratam de questões genéticas, existem outros atores importantes nesse contexto. É o caso dos RNAs. Para que a mensagem do DNA seja acessada, ela precisa ser transcrita (ou copiada) na forma de um RNA mensageiro, que vai orientar a síntese de uma proteína.
Durante a transcrição do gene e a produção de proteínas, podem ocorrer modificações na estrutura da molécula de RNA. Esse processo de edição é mediado principalmente por duas enzimas, a RNA editing 1 e a RNA editing 2. Elas modificam a sequência de ácidos nucleicos e, consequentemente, alteram o tipo de proteína produzida – e tudo isso ocorre sem que haja qualquer alteração na sequência do DNA que é o material genético herdado dos pais.
Por bioinformática é possível mapear o total de modificações que ocorreram nas sequências de RNA do paciente, conta à Agência FAPESP Mirian Hayashi https://bv.fapesp.br/pt/pesquisador/8998/mirian-akemi-furuie-hayashi, professora do Departamento de Farmacologia da Escola Paulista de Medicina (EPM-UNIFESP) e coordenadora da pesquisa no Brasil.
“Cada uma dessas enzimas [RNA editing 1 e RNA editing 2] tem um padrão de edição e, ao analisar o sangue, é possível verificar o padrão de edição de RNA do paciente. Como é muito difícil acompanhar o funcionamento das enzimas, verificamos o produto alterado [modificações específicas na sequência do RNA]. Chegamos assim a um painel com oito biomarcadores, que seriam o padrão de edição de RNA capaz de diferenciar os pacientes. Como sabemos qual é o padrão para o transtorno bipolar e para a depressão, é possível usar esses biomarcadores como um suporte ao diagnóstico desse transtorno”, afirma.
Segundo Hayashi, os pesquisadores da França já acreditavam que o padrão de edição de RNA poderia ser um biomarcador para o diagnóstico do transtorno bipolar. “Conforme comprovado em estudos com pacientes franceses. No entanto, por se tratar de uma população muito homogênea, quiseram repetir os mesmos estudos com pacientes brasileiros para validar o que foi visto no país Europeu”, conta.
Novas possibilidades
No artigo, são descritos oito biomarcadores sanguíneos baseados em edição de RNA (PDE8A, CAMK1D, GAB2, IFNAR1, KCNJ15, LYN, MDM2 e PRKCB) que se mostraram capazes de diagnosticar o transtorno bipolar com depressão com alta sensibilidade e especificidade.
“Essa combinação de biomarcadores foi validada em pacientes com transtorno bipolar depressivo e eutímico [que são pacientes em tratamento e com remissão dos sintomas clínicos]. Encontramos mais de oito alvos, mas os citados no artigo são os que dão a melhor resposta para o diagnóstico. A combinação desses oito RNAs é importante”, ressalta João Nani https://bv.fapesp.br/pt/pesquisador/705596/joao-victor-silva-nani, bolsista da FAPESP e coautor do estudo.
Um fato importante é que os pesquisadores ainda não conseguiram identificar se a alteração no RNA é resultado do transtorno ou do tratamento. “A gente discute no artigo que ainda não é possível definir se isso é efeito da doença ou da medicação. Afinal todos os voluntários são pacientes em tratamento”, diz Nani.
Caso se constate que seja efeito da medicação, avalia Hayashi, abre-se a possibilidade de usar o painel de biomarcadores para verificar se o paciente está de fato se medicando.
Segundo Nani, biomarcadores desse tipo poderão ainda, no futuro, ser norteadores de novos tratamentos. Isso porque a edição de RNA é um mecanismo crucial na regulação do sistema imunológico inato e da resposta inflamatória.
“Essas modificações de RNA servem para evitar que ocorra uma resposta imune contra o próprio organismo. As enzimas editing 1 e 2 são importantes para regular mecanismos de resposta autoimune. Já existem estudos sobre câncer que usaram inibidores dessas moléculas associados a quimio e imunoterapia visando aumentar a eficácia do tratamento. Quem sabe um dia essa modulação possa ser aplicada no tratamento de transtornos psiquiátricos”, cogita Nani.
Também assinam o estudo a pesquisadora Elisa Brietzke, atualmente no Queen’s University, no Canadá, Dinah Weissman e Franck Molina, do CNRS.
O artigo “Euthymic and depressed bipolar patients are characterized by different RNA editing patterns in blood” pode ser lido em https://www.sciencedirect.com/science/article/pii/S0165178123003724?via%3Dihub.
Mais informações em: https://agencia.fapesp.br/estudo-identifica-biomarcadores-que-poderao-auxiliar-no-diagnostico-da-bipolaridade-depressiva/52399


